How to Read Western Blot
Western blotting is a powerful lab technique that helps scientists study proteins, especially their size, abundance, and modifications. It’s widely used to detect specific proteins in a mix of proteins and provides essential information for medical, biological, and chemical research. But if you’re new to it, understanding how to read Western blot results can seem a little tricky. Let’s break it down step-by-step so you can confidently interpret Western blot data and make it a reliable part of your research process!
What is a Western Blot?
At its core, a Western blot is a method to detect specific proteins within a sample. By separating proteins according to their size and marking them with antibodies, scientists can observe the “bands” of protein on a membrane. These bands indicate where the proteins are and help measure their presence or concentration in a sample.
The Western blot process was originally developed by scientists (Towbin and Burnette) and has since become a fundamental tool in molecular biology. Let’s walk through how the process works and how you can analyze and understand the results.
The Key Steps of Western Blotting
Western blotting is composed of a few main stages:
1. Sample Preparation
Start by preparing your sample—whether it’s a cell lysate or a protein sample you want to study. Isolate the proteins from the sample and load them onto a gel.
2. Electrophoresis
Run the sample through a gel to separate proteins by size, usually with a method called SDS-PAGE. Once separated, the proteins appear as bands, which makes it easier to identify individual proteins based on their molecular weight.
3. Transfer to a Membrane
Transfer the separated proteins onto a membrane (such as a nitrocellulose or PVDF membrane). This step, known as blotting, uses an electric current to move proteins from the gel to the membrane.
4. Blocking
Block any nonspecific sites on the membrane so that antibodies only bind to the proteins of interest and not the surface of the membrane. A blocking buffer, typically made with BSA, is commonly used here.
5. Antibody Incubation
Add the primary antibody, which binds to the target protein. Follow up with a secondary antibody that binds to the primary antibody and helps detect the target protein through signals like chemiluminescence or fluorescence.
6. Detection
Visualize the target protein on the membrane. The secondary antibody is typically linked to an enzyme that emits light when exposed to a substrate, allowing you to see the protein band.
7. Imaging
Capture an image of the membrane using a CCD camera or scanner. This image shows the bands for each protein, which can be analyzed. For more information about how to optimize your image capture step you can read our guide here.
How to Read Western Blot Results
Reading and analyzing Western blot results requires comparing band sizes, understanding band intensity, and calculating protein concentration. Here’s how to break it down:
1. Confirm Protein Identity with Molecular Markers
Compare your protein bands to molecular weight markers on the membrane to confirm the size of each band. These markers help you identify if the band corresponds to your protein of interest by aligning it with known sizes.
2. Assess Band Intensity
The intensity (brightness) of each band provides clues about the amount of protein present in your sample. For instance, a brighter band typically means more protein, while a faint band suggests lower abundance.
3. Stay in the Linear Range
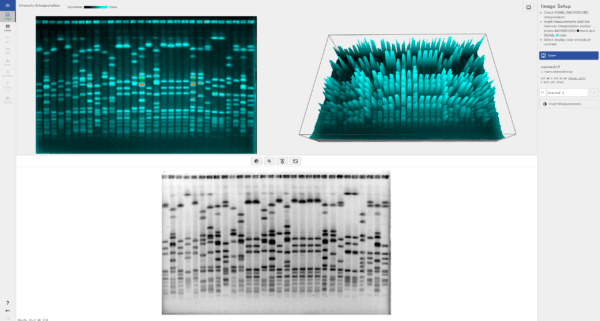
To ensure accurate quantification, stay within the “linear range” of detection. The linear range is where the amount of protein corresponds proportionally to the band intensity. Outside this range, the signal may become saturated, making it impossible to measure accurately. TotalLab’s 3D gel view allows users to quickly and easily check if their protein concentration has exceeded the dynamic range of the instrument they’re currently using.
4. Normalize Your Data
To avoid variability, compare your results to a control band, often a “housekeeping” protein like actin, GAPDH or tubulin. This comparison helps standardize the data, ensuring more reliable results across multiple samples.
Tips to Improve Western Blot Readability
- Avoid Saturation: Dilute samples and adjust exposure times to prevent overly bright bands.
- Reduce Background Noise: Background noise can affect clarity, so use software tools to remove it for more accurate results.
- Use Quality Reagents: High-quality antibodies and reagents improve the clarity of your bands and overall result quality.
FAQs About Western Blot
What does a Western blot tell you?
A Western blot reveals the presence, size, and abundance of specific proteins in a sample. It’s useful for studying protein modifications, protein-protein interactions, and differences in protein levels under various conditions.
How long does a Western blot take?
A typical Western blot can take several hours to a full day, depending on the complexity of the protocol and antibody incubation times. In some cases, results can be processed more quickly.
Why is blocking important in a Western blot?
Blocking prevents antibodies from binding nonspecifically to the membrane, ensuring they only attach to the target protein. This step reduces background noise, making the bands easier to read and analyze accurately.
What is the purpose of normalization in Western blotting?
Normalization helps control for experimental variation by comparing target protein bands to control protein bands (housekeeping genes) that remain constant throughout the experiment. This comparison standardizes the results, improving reliability.
What is the difference between Western blot and ELISA?
While both Western blot and ELISA detect proteins, Western blotting separates proteins by size and identifies them with antibodies on a membrane, making it ideal for protein analysis. ELISA is generally faster and works well for high-throughput detection of antibodies or antigens.
FAQs about Totallab
What does Totallab offer?
Totallab provide advanced software for analyzing and interpreting results from Western blots and other gel-based assays. Their tools make it quick and easy to automatically quantify band intensity, measure protein concentration, and reduce background noise.
Can Totallab software help with Western blot analysis?
Yes! Totallab’s Phoretix 1D software is designed specifically for Western blot quantification, with features such as background subtraction, normalization, quantity calibration and linear range detection. This helps ensure accurate data interpretation and minimize inter-operator error.
How can I get Totallab’s software?
You can visit Totallab’s website to explore their software offerings and request a demo or quote. They provide options for labs of all sizes, making high-quality analysis accessible to researchers worldwide.
Western blotting is a versatile tool that, when read accurately, provides valuable insights into protein function and interactions. Whether you’re new to Western blotting or refining your technique, mastering these steps can help you obtain clear, reliable data for your research. For more advanced analysis, tools like Totallab’s software are great resources to streamline and automate your workflow!
