Automated HCP ELISA Analysis Software | SpotMap ELISA
Current Challenges in ELISA Based HCP Analysis
- Slow software. Difficult to drive and time-consuming, the current gold standard software supplied with plate readers by OEMs is out of date with current OS versions and slow to process results.
- Difficult to observe long term trends. Current software packages focus only on experiments in isolation, meaning if you want to observe how your standards or samples are changing over time it’s up to you to perform this manual work in an external program.
- Lack of automation. Despite the maturity of ELISA as an analytical technique, data analysis still remains a time-intensive and laborious process. SpotMap ELISA is designed as a “set it and forget it” solution automating your analysis using customizable templates that can be easily shared across users or sites.
- 21 CFR/GMP-compliance. When combined with our AuditSafe software, allows users to meet and even exceed FDA 21 CFR part 11, EudraLex Annex 11, GAMP4 or more general GMP standards/ALCOA principles for compliance within regulated or QC/QA environments, from exported .CSV file all the way through to final analysis and report production.
The software has been developed in collaboration with and in response to pharmaceutical industry feedback, it is easy to master, with accurate results obtained quickly and reproducibly.

Harmonize Your Lab Equipment
SpotMap ELISA is designed to accept the industry standard .CSV file type available as an export from almost every plate reader used in the industry. By using our device agnostic software users can reduce capital expenditure costs and the training burden of incorporating multiple devices within their workflow. SpotMap ELISA is compatible with all of your existing laboratory equipment and any additional equipment that may be gained during future business acquisitions.

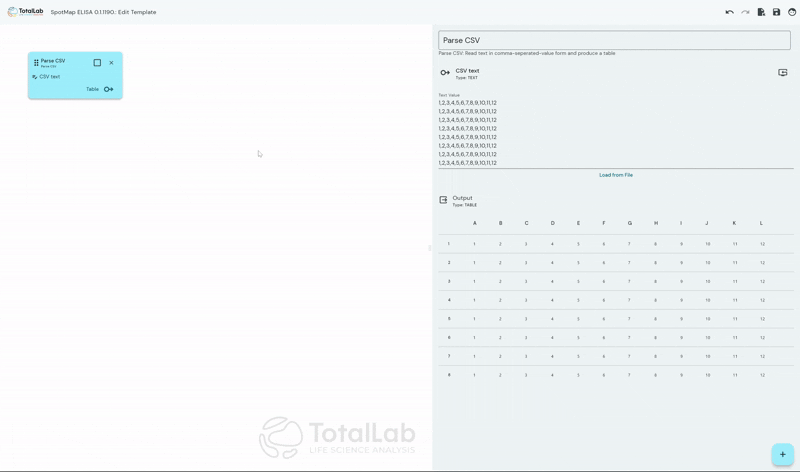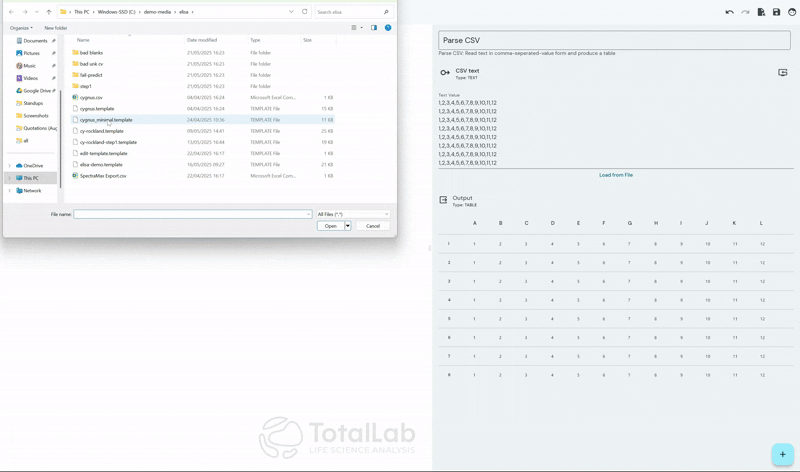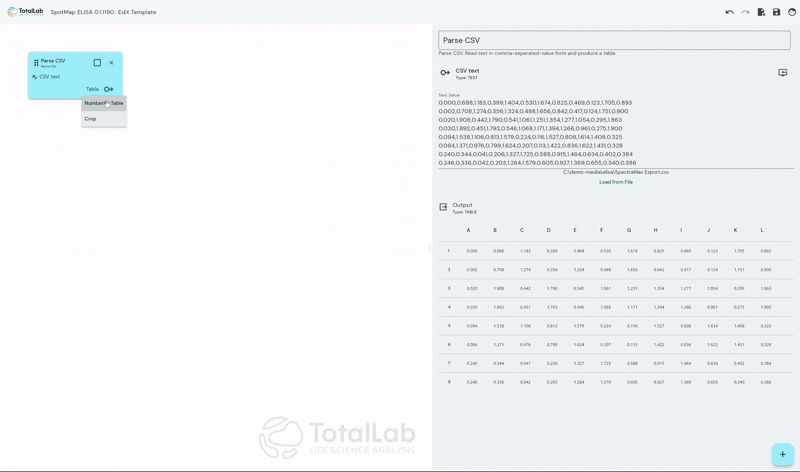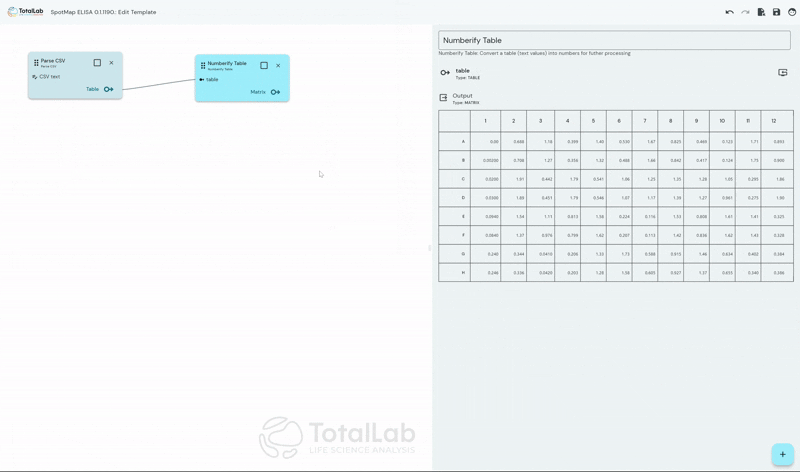
Standardize Your Analysis Process Worldwide
SpotMap ELISA facilitates incredibly customizable automatic analysis of ELISA data by allowing users to create node based, mathematical processing templates. Once these templates have been configured, when it’s time to analyze your next run simply load your template and your .CSV file and go straight to your results with no need for manual intervention. This advanced automation saves users a huge amount of manual number crunching in something like Excel, significantly reduces reduces the opportunity for user error and reduces inter-operator variability.
Users can build up an unlimited library of templates to cover all their routine analyses and then share them easily with colleagues or even other sites anywhere in the world via something as easy as an email attachment to ensure they’re all running exactly the same analysis settings. This removes the potential for operator error entirely, greatly reduces inter-operator variability therefore reducing risk, harmonizing multiple global production or research sites and for tech transfer to a CDMO/CMO.


21 CFR Part 11 and GMP/GMP-compliance
SpotMap ELISA, along with all TotalLab software, can be integrated into our 21 CFR Part 11/GMP-compliant AuditSafe software to ensure full 21 CFR/ALCOA/GMP/EU Annex 11 compliance for use in regulated industry environments (such as the pharmaceutical industry) where data traceability, accountability, and integrity are critical.
AuditSafe restricts software and data access through secure user sign-ins, granular permissions, audit logs, electronic signatures and image authenticity checks. Click here to find out more.
Full IQ/OQ Validation Packages Available
At TotalLab, we’ve been supporting the world’s largest pharmaceutical companies with highly secure, production-ready versions of our software for over a decade now and enjoy very good relationships across the industry.
We know the importance of properly validated software and how it forms part of your QC journey so we offer a full, in-person IQ/OQ package to accompany the sale of regulated versions of our software
Flexible Licensing Options
At TotalLab we’ve been working with pharmaceutical and academic life science customers for decades, we know that some of our users prefer offline licensing to enhance security within their sites. This isn’t a problem, all TotalLab software can be activated easily either online or offline.
Our licensing system licenses a whole computer at once, with no restrictions on the number of users who have access to the software on that computer.
Licenses can be purchased singularly for one computer or we also offer discounts on multiple license purchases at the same time if you want to cover multiple computers across your site; enabling hybrid working to perform analysis outside of the lab or to allow multiple analyses to happen at the same time.
We can also support enterprise licensing for full site-wide licenses covering large pharmaceutical manufacturing facilities and research facilities at a significant discount vs buying singular or multiple licenses.
For pricing and any other licensing related queries please contact us.
How to Purchase
The best way to ensure you’re purchasing the latest version of SpotMap ELISA with the highest level of technical support is directly from us here at TotalLab by using the contact us form. We can accept payment in GBP (£), USD ($) or EUR (€).
We do appreciate that some of our customers prefer sourcing products locally in a local currency or prefer to deal in their native language which may not be English. To facilitate this, TotalLab work with a global network of authorized distributors which you can find here.

