Can I install on a machine with no internet connection?
Yes! all TotalLab software can be activated completely offline and does not need to connect to the internet to maintain the license to facilitate our customers with stricter security requirements in the pharmaceutical industry
Can I use the software on multiple computers?
A single electronic license is tied to one computer, however you can have unlimited Windows accounts on that computer with access to the software. If you do require multiple licenses for multiple different computers or for staff working from home, please let us know using our contact us form and we'd be happy to apply a favorable discount for multi-license purchases.
Which Operating Systems can I use?
CLIQS 1D Pro is compatible with and fully tested under Windows Vista, Windows 7 and Windows 8 and 10, 32 and 64 bit versions.
I have further questions. Can I have a demonstration?
Yes we can organize an online demonstration with a member of our team if required. If you have your own images already then they can be used in any analysis we do. We are always interested in our customers questions and workflows.
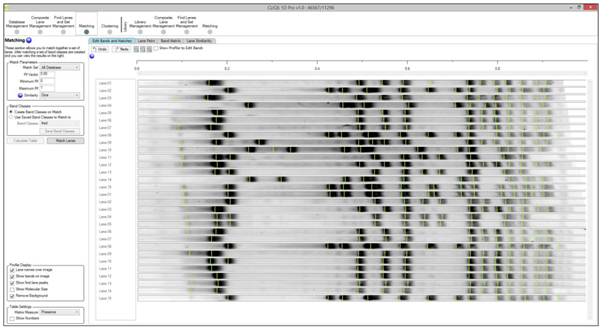
Why does Automatic Lane Detection fail on my gel?
There could be a number of reasons this happens. It could be because
- The lanes are short or have very few bands
- The lanes are too close together so the algorithm cannot split them
- The lanes are not quite the same width which is an assumption the algorithm uses
- The resolution of the image is very low and there is not enough band information, using an AOI for lane detection can help (just draw a box when in automatic detection mode) or simply try to create the lanes manually.
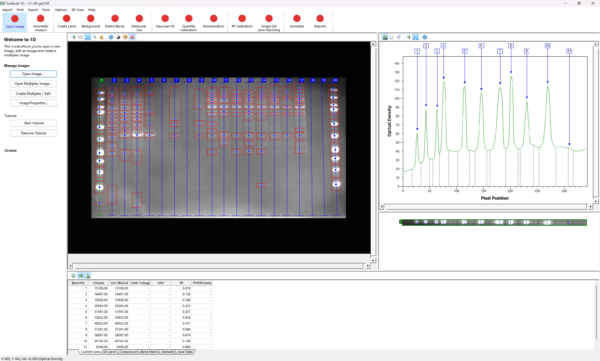
How does Molecular Sizing work?
Each lane has its own calibration curve calculated from where the iso-molecular weight lines(yellow) cross the center of a lane. With only one standard lane these curves are usually the same as the lines just run horizontally across the gel. However with more than one standard lane it joins up the bands of the same value and so the lines can be slightly off horizontal depending on the gel.
Why use different curve types?
The default curve fit is cubic spline. This curve type creates a smooth curve passing through all points. It has no defining equation. This option can be somewhat over sensitive to inaccurate mappings but will guarantee that the results are as expected from where the iso-molecular weight lines(yellow) cross the lanes. First order Lagrange is similar but uses straight lines.
The other main option used historically is the Linear Log equation which is a straight line calculated from the log of the Molecular Size. This is theoretically correct but can lead to misleading results due to any inaccuracy of the curve fit. Even curves with high R2 results can still have problems.
What is the name of the curve fitting algorithm?
We use an iterative algorithm called Levenberg-Marquardt to get the results for the curve fitting equations. Cubic Spline and First order Lagrange are also well-defined algorithms.
My image does not look like the image I captured. Why?
On image load, a histogram of all pixel values is created. Then the default auto contrast is created from the value of the histogram after 0.5% of all pixels and at 99.5% of all pixels. This stops outliers or scanner noise affecting the result, but it can look a little different.
If the image has been calibrated or an uses an encoding (from Cytiva or Fujifilm manufactured scanners) then the converted values are used by default for the image. If you do not want this, then use the “Raw” option to go back to the raw pixel values.
Note: Contrast does not affect the values used in any calculations.
My lane profile is upside down. What can I do to fix it?
Go to the File menu and select Invert Measurements to reverse the calculation used to generate the Profile. This option is remembered so you should not need to change it again.
What file formats can the software support?
The software can load in .tif, .tiff, .jpg,.jpeg, .png, .gif, .gel, .img (Fuji), .bmp files.
When I load in my JPG file the image is inverted
This occasionally happens as part of the loading function. Just press the invert button on the toolbar to see if reversed again.
What is Band Volume?
This is defined as the total value of all the pixels in a band with background subtracted. The pixels are shown on the image as between the 2 red lines (the band edges). Another way to look at it is as the area under the profile for the band (with background subtracted) multiplied by the lane width.
Where can you get the total volume of all the bands in a Lane?
This information can be found in the Analysis Report (previously called Gel Report). On the first page total band volume (and total lane volume) are displayed in a summary table of all the lanes in the gel.
What is Profile Deconvolution?
This option allows the user to model each band as a gaussian and use these to recalculate the band volume. This is sometimes used to split the volume of overlapping bands in what is believed to be a more accurate manner. Advanced fitting is best but there are problems. They are firstly, that is can be very slow and secondly, without good background subtraction they can be very wrong.
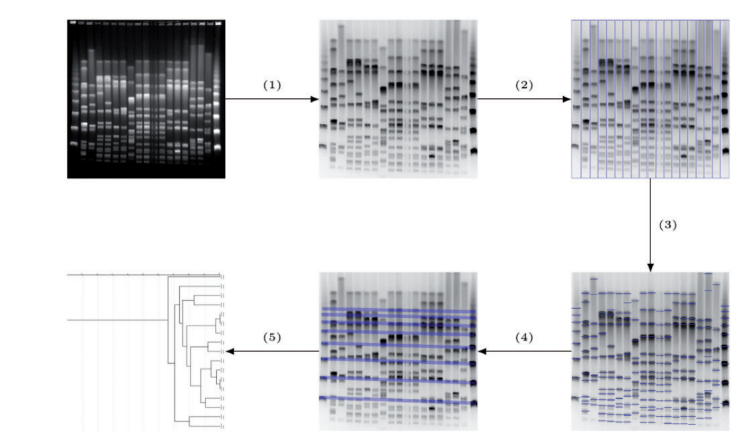
How do I export images/dendrograms in CLIQS 1D Pro?
To do any exporting to the clipboard or to file just right click on the image to bring up a context menu then choose the function you require.
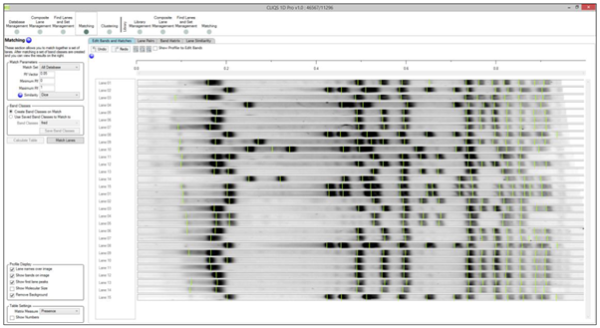
What is the normal procedure for using Rf lines to aid matching?
Use the same marker lanes in every gel (or common bands across the gels)
Put an Rf line on each marker band you wish to use and adjust for any smiling (you do not need to use an Rf line for all marker bands)
Edit the number of the Rf line so they are consistent between gels
Make sure the 0 and 1 marker lines are either in consistent places on each gel or removed so that the values are extrapolated from the other Rf line
I cannot move my Rf lines?
This may be because they are too close to each other. There is a limit to how close they can be and on low resolution images you may not be able to move them close to each other.
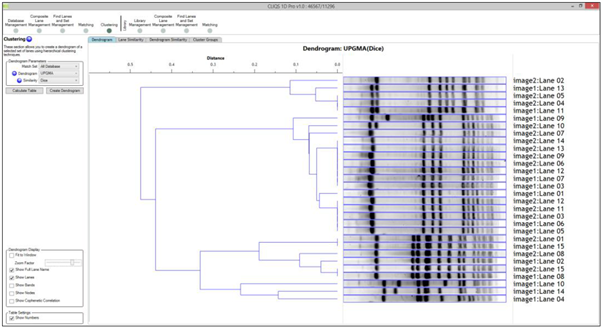
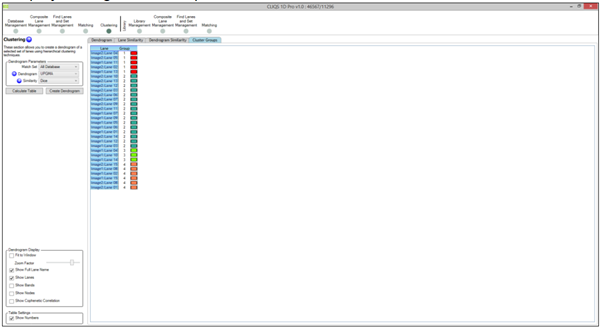
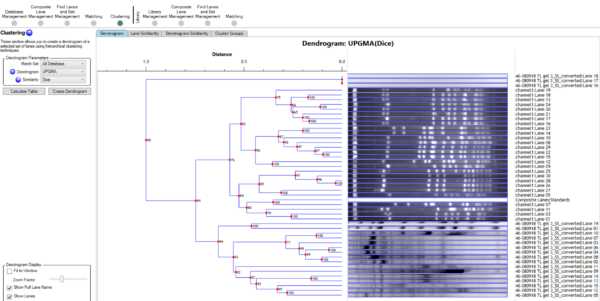
In 1D can I select which lanes to do a Dendrogram on?
Yes. Standard lanes are not matched by default and they would not be in a dendrogram (they can also be matched if required). Also, you can use Lane Selection in the Options dialog to switch off some lanes if you wish.
My profile is flat at the baseline, but I can see some background on the image?
This is probably due to Intensity Calibration errors. If your calibration curve intersects the y intersect at too high a value, then all raw values under the y intersect value will be calibrated to zero. This can lead to the background and some lane material showing as zero. Recalibrate the curve to improve your results or force it to go through the origin.
How many lanes can be stored in a database/library?
The limit is only based on RAM and disk space. We have loaded 500 in simple tests and they should cope with >1000.
Can more than one person access the database at the same time?
As many people as they like can access the database at the same time though it has not been designed for this type of use. However, if one user adds lanes to a database/library or changes bands then the other users may not get to see the changes straight away unless they load the database back in. So if you are careful it should be fine.
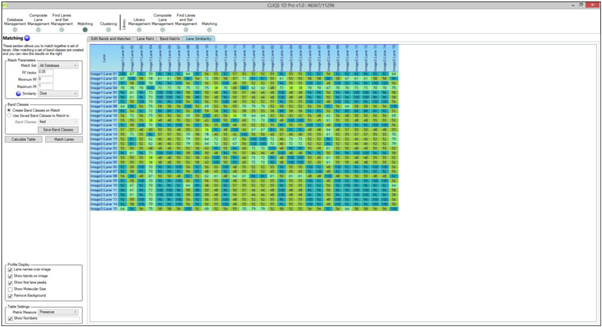
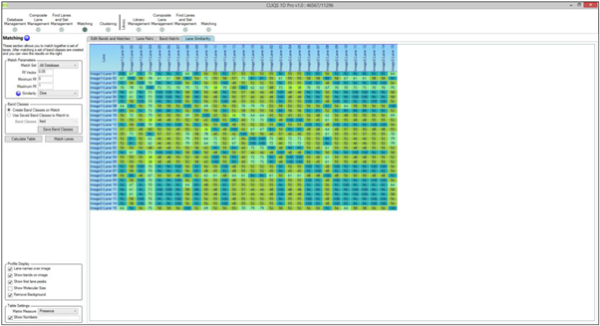
What is the BSI?
BSI is short for Band Sharing Index. It is calculated as (2*number of bands matched)/(total number of bands in both lanes) So for example two lanes have 5 bands and 7 bands. 3 bands are matched in each lane so the BSI = (2*3)/(5+7) = 0.5. This is then displayed as a percentage so it is 50%. It is a measure of how similar two lanes are and is best known as the “Dice” similarity.