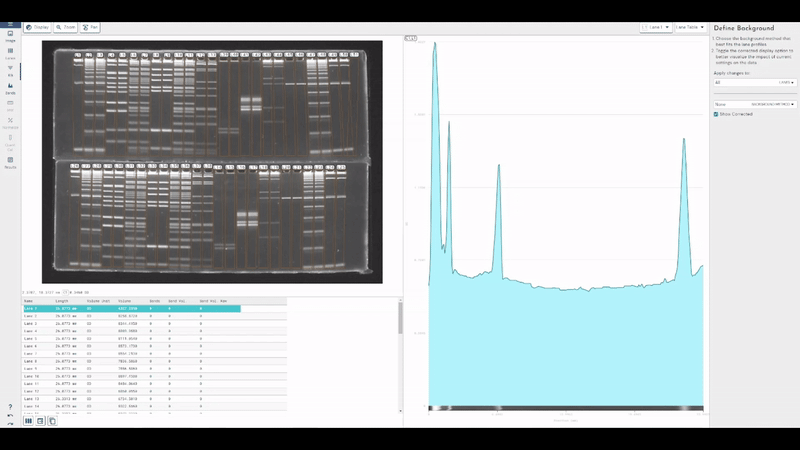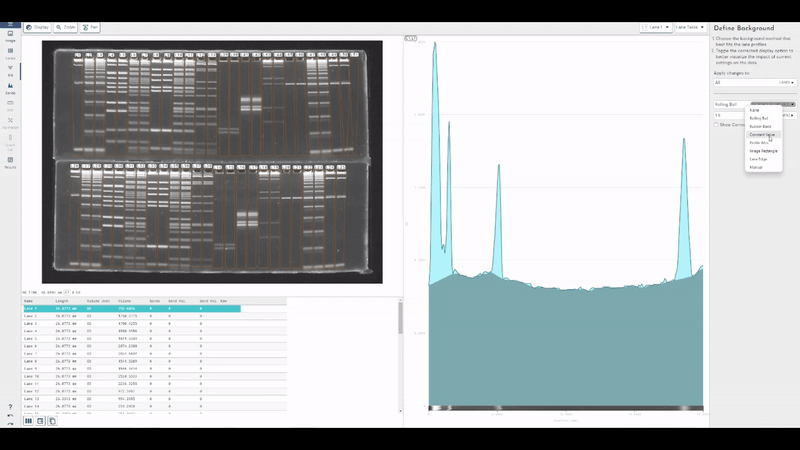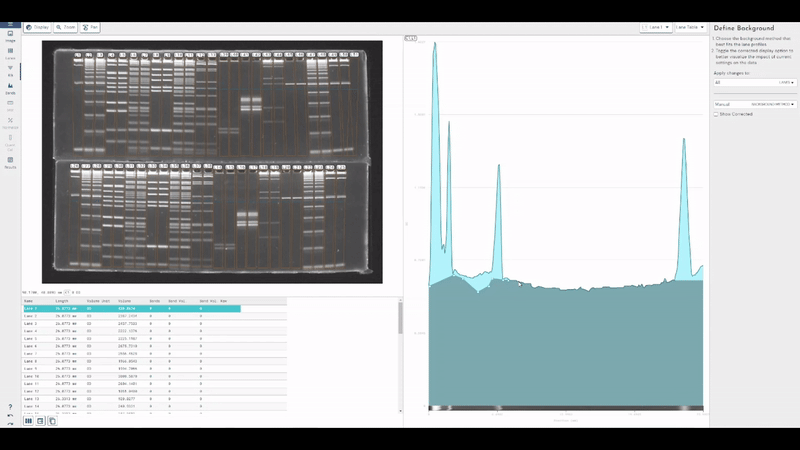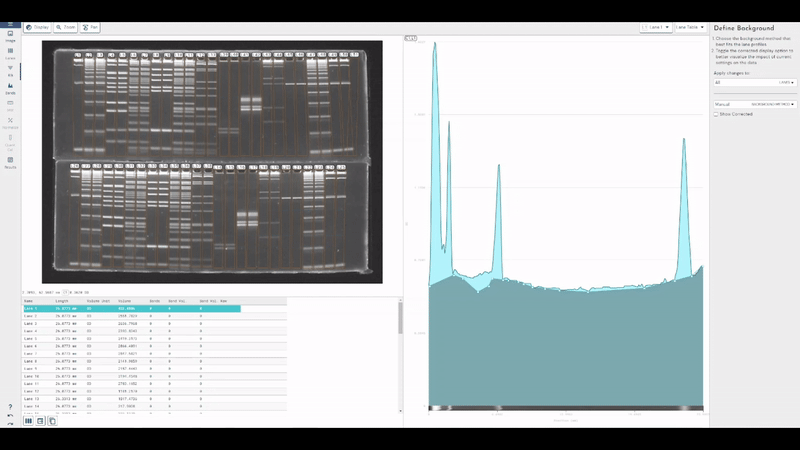
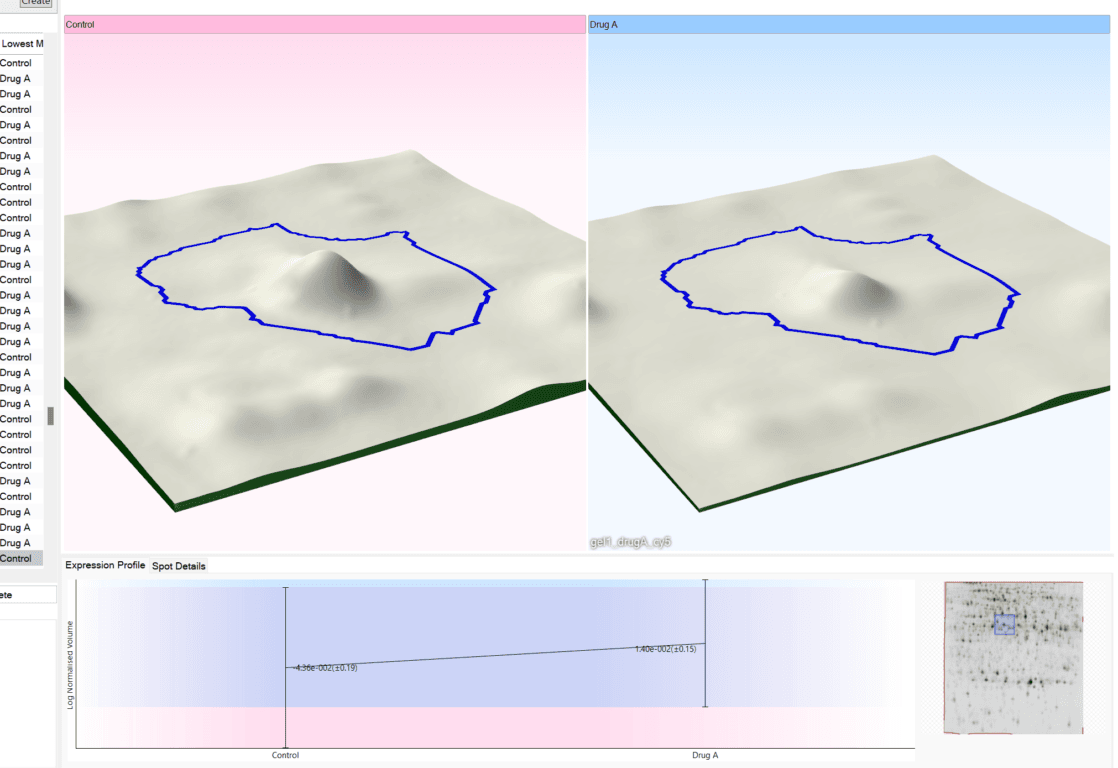
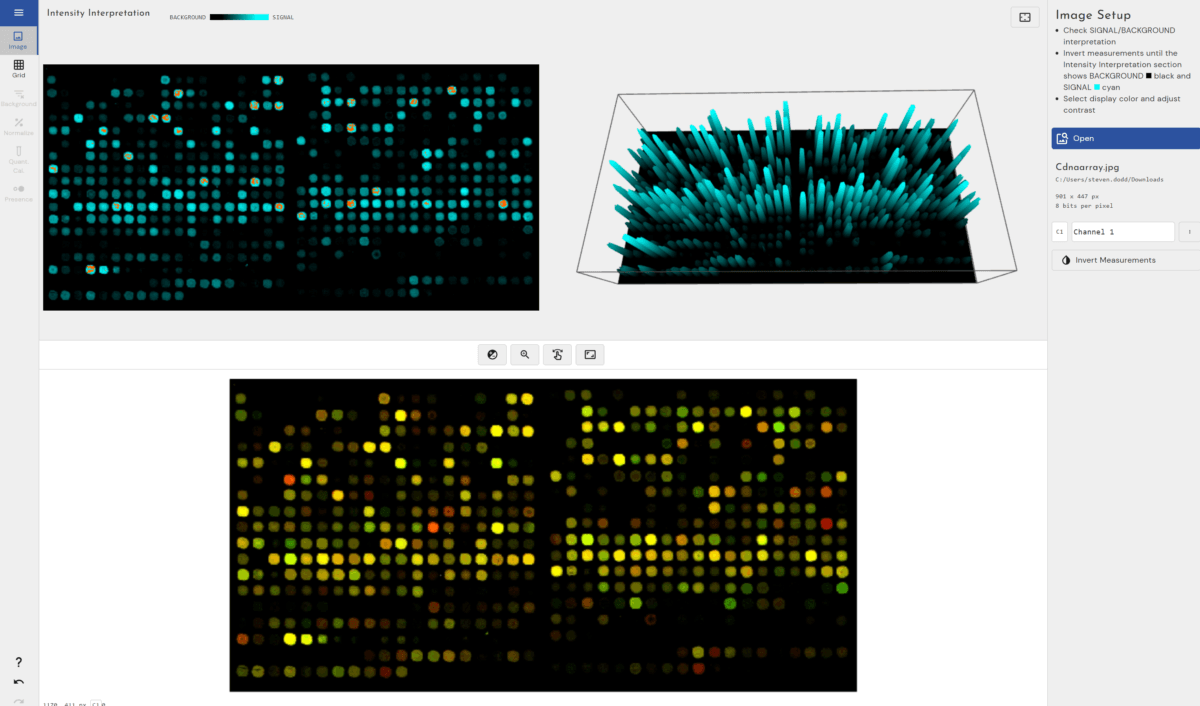
TotalLab joins Scientist.com as a trusted custom software and OEM development partner for big pharma
TotalLab has joined Scientist.com as a trusted custom software and OEM development partner for big pharma, making it simpler for R&D, QC and digital teams to procure compliant, lab‑ready software.
Read More >