SDS-PAGE and Western Blot Image Capture Guide

SDS-PAGE and Western Blot Image Capture Guide
Good image capture is critical to guarantee optimal performance of automated image analysis packages and generate reliable scientific data. This technical document provides a brief guide to the range of different image acquisition devices currently in use for gel applications, defines some the important technical factors required to generate digital images of a quality suitable for automated image analysis and recommendations for producing high quality 1D and 2D gel images for use in our Phoretix 1D, CLIQS 1D Pro, SameSpots or SpotMap software.
This guide includes:
- Recommendations for capturing the best possible images.
- What file formats you should use in image analysis.
- Which type of scanner you should be using.
- What considerations should be made when capturing a gel image.
- Best practices and what should be avoided.
- A checklist to create high quality images.
Checklist
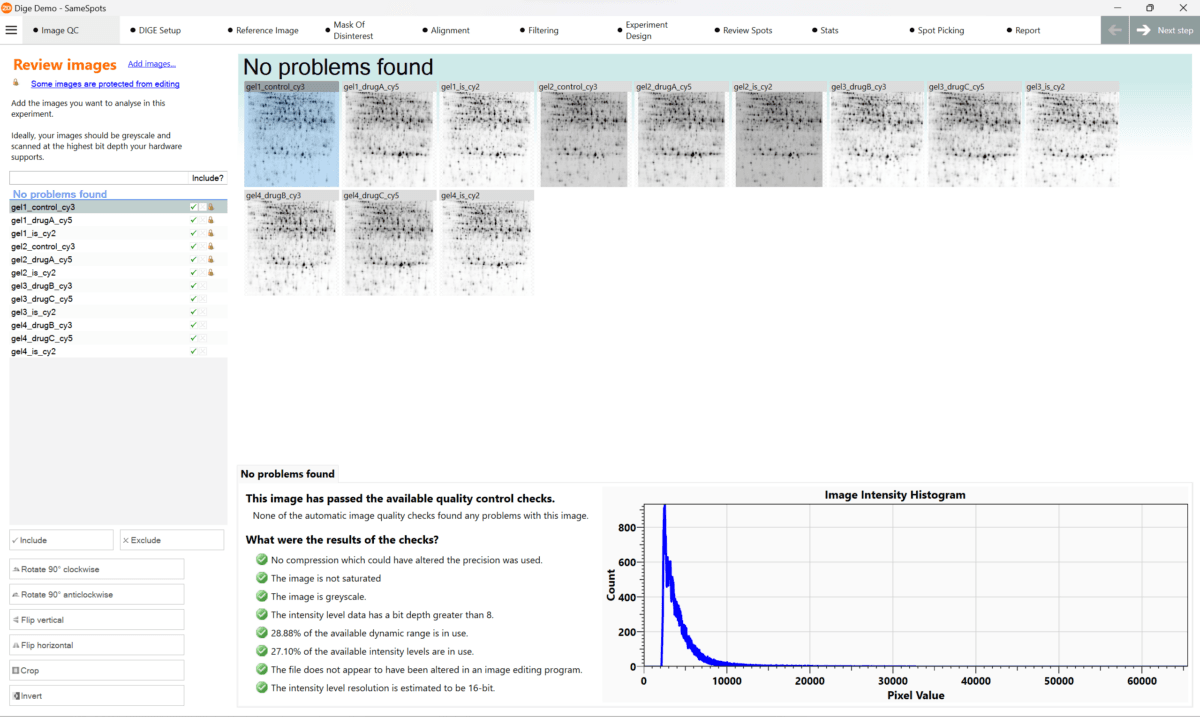
If you want to create good quality images from gels and Western blots here is a summary of all the areas you need to consider in an image analysis SOP with more details below.
- Capture images as grayscale.
- Use the highest possible bit depth. We recommend a 16-bit image file, as it has a resolution of 65536 possible grayscale values for each pixel.
- Set an image resolution such that having a single pixel gap between spots does not affect the spots themselves.
- Attempt to get a high dynamic range to get the best intensity data from the images.
- If possible, only create an image of a region of interest so you are only using dynamic range on the gel.
- If you go too far optimizing the dynamic range it can result in image saturation. This can also occur due to the dye.
- Make the imaging process reproducible by using the same imaging parameters for all gels or blots in your study.
- For multiplex imaging adjust the parameters for each dye separately (
- Never use compressed images, such as .JPEG files, for quantitative analysis.
- If possible, use GEL or IMG/INF file formats, as they often contain additional grayscale calibration information. However a 16-bit TIFF file is the most widely used file format for image analysis.
- Process all gel images using the same orientation.
- Do all image editing in your analysis software (this functionality is available in all TotalLab analysis software) as this does not affect any image quantification.
- Limit image editing to crop, mirror, and rotation by 90, 180, or 270 degrees, this is because rotations between multiples of 90 degrees are actually approximations by software and not “true” data from your experiment.
- Avoid using third party imaging software like Photoshop for image processing. Since they allow direct modification of the images, image editors could also be used to bias (or even falsify) the experiment data.
Imaging Devices
Image acquisition can be achieved using a variety of devices. These can be broadly categorized into three major types:
- Laser Scanners
- CCD Camera Systems
- Document Scanners
How is the digital image generated?
In general, image capture devices work by illuminating the gel and recording the light emitted from each point. Each of these points is called a pixel. The illuminating light may be either transmitted or reflected by the gel to the detector which converts the light level into an electrical signal. This analogue signal is then converted by an A/D (analogue to digital) converter to a digital number for each pixel. Pixels are then combined to produce a digital image of the entire gel. In addition to transmission and reflection, a third possibility, fluorescence, occurs when the illumination is used to excite molecules bound to the proteins in the gel. These molecules fluoresce and the emitted light is detected in the same way as for transmission and reflection.

Laser Scanners
Laser devices are the most sophisticated and versatile image acquisition instruments, and are commonly used to detect some of the more recently developed fluorescent dyes such as Cy dyes, Sypro, ProQ and Deep Purple. Powerful laser(s) set at a specific wavelength(s) scan the gel, point by point, and the resultant emission energy is detected by high voltage photomultiplier tubes (PMTs) and converted into digital signal (pixels). Multiple lasers and emission filters can be used to accommodate the wide variety of fluorescent dyes currently available. Some instruments also benefit from confocal optics, which exclude signals from scattered light, thus enabling gels to be scanned whilst between low fluorescence glass plates. This feature is particularly useful for DIGE applications. Laser-based image capture devices can also be used with the common visible protein stains such as silver and Coomassie Blue, and also for phosphor-imaging of radioactive labelling.
CCD Camera Systems
CCD camera image acquisition systems can be used with either visible dyes or fluorescent stains. These instruments operate with visible or UV illumination for visible protein stains, and fluorescent or Xenon lamps for fluorescent applications. The emitted light is captured by high sensitivity cooled area array CCD sensors and converted into digital signal. The CCD cameras can be either fixed or scanning. Scanning cameras are used to compensate for the relatively low dimensions of high quality camera chips (typically less than 2000 x 2000 pixels), and function by generating a series of overlapping images, which are assembled to form the final image. Some instruments use different modes of illumination; coming from the top (for fluorescent dyes such as Sypro), bottom (for visible dyes) or edge of the gel assembly. The latter facilitates DIGE applications, as it enables gels to be scanned whilst between low fluorescence glass plates. Although CCD is currently the most popular sensor device used in cameras, Complementary-Metal-Oxide-Semiconductor (CMOS) devices are emerging as an alternative, offering a number of advantages, including a broader dynamic light range.
Document Scanners
Standard commercial document scanners are often used as densitometers. In newer scanners, the light source is either a cold cathode fluorescent lamp (CCFL) or a xenon lamp, while older scanners may have a standard fluorescent lamp. The gel is illuminated and the resultant reflected or transmitted light is detected, line by line, as electrical current by linear array CCD sensors and subsequently converted, into digital information. Flatbed scanners offer both transmittance and reflectance, and are used for imaging visible dyes like silver and Coomassie, and to scan autoradiographs or blots. In general, the scanners used for gel applications differ from commercial office scanners in that their optical path is modified to cope with the gel assembly and they are sealed units to protect against wet samples.
| Laser Scanners | CCD Camera Systems | Document | ||
|---|---|---|---|---|
| Scanning | Fixed | |||
| Image Resolution | 10-250 | 50-200 | >120 | 20-250 |
| Dynamic Range | 5 | 3-4 | 3-4 | 4-5 |
| Scan speed | slow | slow | medium | fast |
| Wavelength | high | high | high | low |
| Silver, Coomassie autoradiography |
yes | yes | yes | yes |
| Storage phosphor | yes | no | no | no |
| Single color fluorescence (Cy dyes, Sypro, Deep Purple, ProQ) |
yes | yes | yes | no |
| Multicolor fluorescence (DIGE) |
yes | yes | limited | no |
| Chemiluminescence | yes | yes | yes | no |
| Cost | very high | high | medium | low |
Image Acquisition – the Practicalities
There are a number of important considerations that should be made when capturing a gel image. These include:
- Bit depth
- Spatial resolution
- Dynamic range
Inadequate resolution in any of these may cause sub-optimal detection and may also compromise quantitative results when using any image analysis software.
Grayscale or Color
If the software loads a color image it is automatically converted to grayscale for analysis. A color image is made up of 3 different channels; Red, Green and Blue. So a 24-bit color image has 8-bits per channel. When the image is converted to grayscale, it’s effectively changed to an 8-bit grayscale image with weighted conversion of the Red, Green and Blue channels. So a colour image, when imported to the software, will lose information and be converted in a way that may not be accurate for the image capture device used.
Recommendation:
Always scan directly to grayscale as the imaging device will then do the conversion from color in the most accurate and sensitive manner. It will also allow for a higher bit depth.
Bit Depth
Also referred to as color depth or pixel depth, bit depth is the number of bits used to represent the grayscale (intensity level) of each pixel in an image. Greater bit depth allows a greater range of shades of grey to be represented by a pixel. For example, an 8-bit grayscale image file stores 256 shades of grey for each pixel, while a 16-bit image file has 65536 possible grayscale values for each pixel. The following table indicates the possible grayscale levels available for the types of images commonly used for gel image analysis.
| Bit Depth | Intensity Levels |
|---|---|
| 8 | 256 |
| 10 | 1024 |
| 12 | 4096 |
| 16 | 65536 |
In reality, the images displayed on the computer screen will only be represented in 256 shades of grey, and so an 8-bit image will look identical to a 16-bit image by eye. However, image analysis software can distinguish between the different levels of grey.
Recommendation:
As a rule, the more levels of grey represented in an image, the better the ability to differentiate low abundance spots from the background, and the greater the quantitative accuracy. This is further illustrated in Figures 1 and 2, comparing spot detection in an identical area on the same 2D gel, captured at 8-bit and 16-bit.

Figure 1. Spot detection on an 8-bit image (a) image view and (b) 3D view. Two low level spots are clearly undetected. (c) A profile through one of these undetected spots shows it to have a maximum pixel intensity of 33, which is only 9 grey levels above background.

Figure 2. Spot detection on the same 2D gel image, but captured at 16-bit (a) image view and (b) 3D view. The two spots which were previously below the limits of detection for the 8-bit image are now clearly well detected. (c) A profile through one of these spots shows it to have a maximum pixel intensity of 8390, which is 2062 grey levels above background for this image.
Recommendation:
As a rule, the more levels of grey represented in an image, the better the ability to differentiate low abundance spots from the background, and the greater the quantitative accuracy.
Image Resolution
Image (or spatial) resolution relates to the number of pixels displayed per unit length of a digital image, and is often measured in dpi (dots per inch) or in microns (the size of the area each pixel represents). Images with a higher spatial resolution have a greater number of pixels and have more image detail than those of lower spatial resolution. It is important to be aware that variations in spatial resolution will not only affect the final appearance of the image, but will also impinge on the quality of spot detection and the accuracy of any subsequent quantitative measurements. At low resolutions, there will be fewer pixels available to represent each spot, and as a result, spot detection and quantitative accuracy will be compromised.

Figure 3. Spot detection on a 20cm 2D gel image, captured at a resolution of (a) 100dpi and (b) 300dpi. The lower resolution of the 100 dpi image is apparent by the degree of pixilation. In this image, there are fewer pixels present to represent the spots (approximately 2 orders of magnitude less pixels in the 100 dpi image compared to the 300 dpi image).
As a result, spot detection may be compromised, particularly in highly populated areas where spots may only be separated from one another by a single pixel. In these situations, a spot outline cannot be accurately placed, and one spot may end up “losing” material to its neighbor, as illustrated in the 3D representation of spot detection for the 100 dpi image. In contrast, these problems are not encountered when the image resolution is increased to 300 dpi. Furthermore, higher resolution means that more pixels, and hence, more data, are available for the analysis, with a result that quantitative measurements will be more reliable.
There is, however, a maximum resolution which once exceeded produces minimal additional information. Once you have sufficient resolution to adequately represent the smallest features, any further increases in spatial resolution will simply increase the accuracy with which you can represent the noise in the system. In addition, every doubling in spatial resolution quadruples the amount of data that has to be processed which can cause problems in processing speed and memory management. The following table shows the variation in pixel content and file sizes of a 20cm gel image, captured at different image resolutions and bit depths.
| Resolution (dpi) | Resolution (micron) | Image Dimensions (Pixels) | Total no. of pixels per image) | Bit Depth | File Size (Mb) |
|---|---|---|---|---|---|
| 100 | 254 | 776 x 775 | 5.97 x 10⁴ | 8 | 0.6 |
| 200 | 254 | 1553 x 1550 | 2.41 x 10⁶ | 8 | 2.7 |
| 300 | 84.67 | 2329 x 2325 | 5.41 x 10⁶ | 8 | 5.3 |
| 100 | 254 | 776 x 775 | 5.97 x 10⁴ | 16 | 1.2 |
| 200 | 254 | 1553 x 1550 | 2.41 x 10⁶ | 16 | 4.8 |
| 300 | 84.67 | 2329 x 2325 | 5.41 x 10⁶ | 16 | 10.6 |
The following table shows the variation in pixel content and file sizes of a mini gel image (approximately 7 x 5 cm), captured at different image resolutions and bit depths.
| Resolution (dpi) | Resolution (micron) | Image Dimensions (Pixels) | Total no. of pixels per image) | Bit Depth | File Size (Mb) |
|---|---|---|---|---|---|
| 100 | 254 | 290 x 22 | 6.38 x 10³ | 8 | 00.06 |
| 300 | 84.67 | 871 x 65 | 5.66 x 10⁴ | 8 | 0.06 |
| 600 | 42.3 | 1742 x 1310 | 2.28 x 10⁶ | 8 | 2.2 |
| 100 | 254 | 290 x 22 | 6.38 x 10³ | 16 | 0.01 |
| 300 | 84.67 | 871 x 65 | 5.66 x 10⁴ | 16 | 0.1 |
| 600 | 42.3 | 1742 x 1310 | 2.28 x 10⁶ | 16 | 4.5 |
It is important to note that, in order to achieve equivalent pixel information in a 7 x 5 cm mini gel, compared to a 20 cm gel which has been scanned at 200 dpi, the mini gel must be scanned at 600 dpi.
Recommendation:
Try to scan at the best resolution for your images. In most situations, 300 dpi or 100 microns will provide an image that is large enough for accurate analysis and small enough for efficient processing. However, if your gels are small (e.g. mini gels), then you may need to increase the resolution to achieve this. As a rule of thumb, the active area of the gel (i.e., the area of spot material) should fall in the range 1000-1800 pixels in both horizontal and vertical directions. This range provides a good trade-off in information content and analysis performance.
Dynamic Range
For image analysis, the dynamic range (or grey level resolution) refers to the actual range of grayscale levels being used by the image which will be less than the available range of values. For example: with a 16-bit image there are 65,536 available values and your data will lie somewhere inside that range. It is good practice to optimise scanning so that the majority of the available grayscale range is represented; a limited dynamic range can not only impact on the quality of image analysis, it may also compromise quantitative results when comparing data between images.
Example A: Good dynamic Range (67% of available)

Example B: Low dynamic Range (12% of available)

Figure 4. (a) An example of good dynamic range using linear and log plots of pixel histograms showing 67% of the available grayscale range in use. (b) An example of a low dynamic range using linear and log plots of pixel histograms showing 12% of the available grayscale range in use.
Recommendation:
If possible, only scan the area of the gel you are interested in. Perform any cropping at the time of scanning to remove blank parts of the scanner plate, labels etc. The extra areas provide no useful information, can ‘steal’ dynamic range, distort image statistics and increase storage requirements. You can adjust the dynamic range in CCD camera systems by altering the exposure time, or in a laser based system by fine tuning the voltage of the PMT detector. It should be as high as possible across all your images, without saturating, given you should not change settings between different images in the same study. You should consult your scanner documentation, or contact your scanner supplier for information on how to achieve this.
Image Saturation
When optimizing the dynamic range, it is important to avoid saturation effects. Saturation occurs when grey levels exceed the maximum available. When a spot becomes saturated, any differences in high pixel intensities cannot be resolved, and the spot appears truncated when viewed in 3D (Figure 5).

Figure 5. (a) View of an area of saturated spots on a gel image; (b) and (c), the same area represented in 3D.
Why can saturation be a problem?
The outline detection is not optimized for the unnatural shape of these spots, since the peaks are effectively ‘missing’ in the scanned image, and no reliable quantitation can be done with them.
Recommendation:
If possible, only scan the area of the gel you are interested in. Perform any cropping at the time of scanning to remove blank parts of the scanner plate, labels etc. The extra areas provide no useful information, can ‘steal’ dynamic range, distort image statistics and increase storage requirements. You can adjust the dynamic range in CCD camera systems by altering the exposure time, or in a laser based system by fine tuning the voltage of the PMT detector. It should be as high as possible across all your images, without saturating, given you should not change settings between different images in the same study. You should consult your scanner documentation, or contact your scanner supplier for information on how to achieve this.
Compressed Images including JPEGS
Avoid using JPEG files for image analysis. The JPEG format is what is called a “lossy” compression system; while the images may look the same they aren’t. A great deal of smoothing and averaging may have taken place within the compression process and this will affect
the underlying raw pixel data. Converting a JPEG image back to a TIFF is not a solution; once the image has been compressed in this way, the data has been lost and cannot be retrieved.

Recommendation:
You should scan your images in grayscale at the highest bit depth your hardware supports- usually 16 or 12 bit, then store them in a lossless format such as TIFF. If possible use GEL or IMG/INF file formats, these often contain additional grayscale calibration information. Please consult your scanner documentation, or contact your scanner supplier for information on how to achieve this.
Image Editing
Simple image editing can be used to “fix” the image if the size or orientation is not correct within the software, simple edits such as crop, mirror, and rotation by 90, 180, or 270 degrees can be completed. This does not affect any image quantification. When can image editing be a problem? Image editing programs can directly manipulate the intensity levels of the pixels in your data and change the size of the images. This could result in distortion of the peaks, incorrect calibration spot volumes, or increased variance between images. Since they allow direct modification of the images, image editors could also be used to bias (or even falsify) the experiment data.
Recommendation:
Rotating, flipping, inverting and cropping images can all be performed in our software. The pixel intensities will not be affected by these operations.
Contrast Stretching (available intensity levels)
This can be another artefact from the capture software or an image editing package. This indicates that some form of “Contrast Stretching” or “Histogram Equalization” has been applied to the scanned image. For example: if an image containing 100 intensity levels is stretched to fill an image format capable of recording 400 intensity levels, the image still only contains 100 unique intensity levels (25% of those available).
Why can contrast stretching be a problem?
The image may look to be a higher resolution but the precision has not been improved. Contrast stretched images have pixel intensities that step up and do not improve spot detection or quantification.
Recommendation:
This is normally optional on the capture software and you should not apply any contrast stretching or equalization to the image.
