"TotalLab's 21 CFR implementation is probably the best I've ever seen and administered on-site"
David Morton
Associate OT Specialist
Lonza
Here at TotalLab, we’ve been collaborating with the largest members of the global pharmaceutical industry for over a decade to create a world-leading 21 CFR/GMP-compliant software solution, working with their internal QC/QA departments User Requirement Specifications (URS) to surpass their own stringent internal requirements for compliance far exceeding that outlined in the actual legislation.
Our AuditSafe platform replaces our industry leading TotalLab GxP, CLIQS 21 CFR, SpotMap 21 CFR, Phoretix 1D 21 CFR, CLIQS Version Control etc. variants of previous software to bring them all under one umbrella. It is designed to be combined with our industry-leading 1D and 2D image analysis software, with software developed by our partners or even software from external manufacturers (Microsoft Excel, GraphPad Prism etc.). If you’re interested in this service for your own software or hardware you can learn more here.
AuditSafe is a complete, easy to use 21 CFR/GMP-compliant solution, it offers:


"TotalLab's 21 CFR implementation is probably the best I've ever seen and administered on-site"
David Morton
Associate OT Specialist
Lonza
AuditSafe wraps securely around existing pieces of equipment and software within one entire workflow, no more gaps in compliance when integrating data between different systems or different manufacturers’ software packages.
Why train your staff to use multiple, difficult-to-use compliance systems within the same analysis workflow when they can just be trained in one?


AuditSafe is TotalLab’s answer to one of the major pain points in introducing regulatory compliance in a manufacturing site – difficulty in integrating the solution within your existing network.
Here at TotalLab we’ve been creating and selling FDA 21 CFR Part 11/GMP-compliant software for the pharmaceutical and other regulated industries (such as hospitals) worldwide for years now with an incredibly robust and feature-rich software solution, however the hardest part of the installation and validation of the software on site has always been navigating the existing users network/IT configuration.
From blocked ports to user permission headaches preventing end users from setting up the software themselves without their own IT professionals to hand, we thought there must be an easier way for our users to become compliant.
AuditSafe is available as a pre-validated plug-and-play on-site server or as a software solution that seamlessly integrates with your existing IT network.
AuditSafe provides users with the usability benefits of a pre-validated cloud-compliance solution without having to sacrifice data security, 21 CFR/GMP compliance or risk breaching local data sovereignty laws.
Whilst AuditSafe is not cloud-based, that doesn’t mean users can’t benefit from some of the advanced interface and dashboard options available in cloud-based clients without giving up control over the security of their data.
AuditSafe is designed from the ground up to administer whole workflows and operational pharma sites, not just singular pieces of software or equipment like other 21 CFR/GMP solutions.
When audited, simply export all audit trails from one browser into a single, human readable file, no more guiding FDA inspectors between multiple different computers or file storage systems for hours at a time. Simple, centralised access to all your records when you need it.
The world of 21 CFR/GMP-compliant software within the life science industry is hugely fragmented. Through our own experience in working with a number of global laboratory equipment suppliers and suppliers of analysis software (including our own) we identified a number of issues that can make compliance and QC within labs more difficult than they need to be:
This means that operators need to be trained specifically for every piece of equipment or software within a specific process or workflow with usually very little cross over between manufacturer’s approaches. This problem stems from every manufacturer and software developer approaching the problem of compliance separately and differently and massively increases the on-site training burden and potential risk for user error and broken compliance.
AuditSafe solves this by being a cross software and hardware compliance solution. Within AuditSafe you can connect all the pieces of equipment within your workflow and the software required to analyze the output of that equipment. This means that your operators only need to be trained in how to use one compliance software – AuditSafe – to perform their work safely.
This was an issue we acutely identified in relation to our image analysis software. Whilst we provided excellent 21 CFR/GxP-compliant image analysis software we were often the first step in the workflow where a rigorous audit trail began. This left a large hole in the compliance chain of custody between image creation by the equipment through to transfer into the analysis software during which data could be edited or changed but there would be no way of knowing if they had. FDA Auditors at customers sites rightly asked questions around this section of the workflow which no audit trail or access log protecting it – “How can you be sure the data you performed your analysis on was the data that came off your machine?”.
More often than not, customer’s didn’t have a rigorous answer to this question.
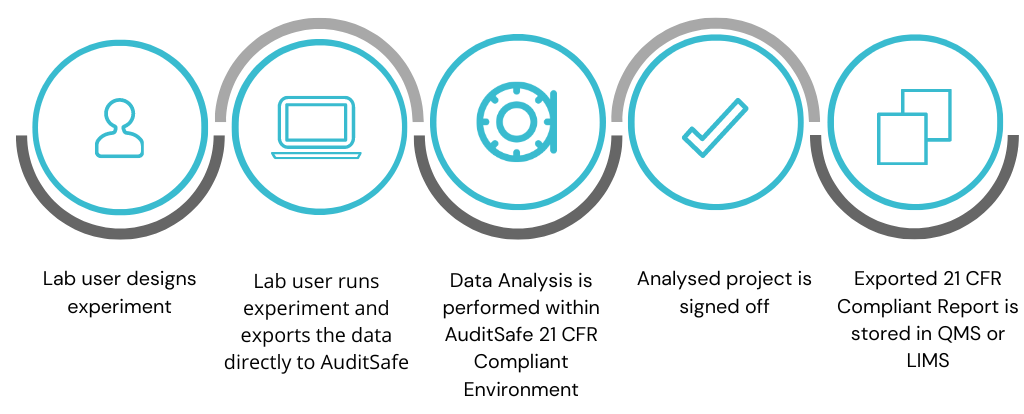
AuditSafe solves this problem by importing raw data from any manufacturer’s equipment at the point of creation, meaning the audit trail, access log and protected access to that data starts as soon as the data exists. This data is then passed, securely, via AuditSafe into the analysis software required to process it. AuditSafe provides a fully compliant, end-to-end solution from raw data through to analysis, report generation and storage within a LIMS.
User accounts limit the features and level of data access within the software that users have access to and is a mandatory requirement of FDA 21 CFR Part 11 Subpart B Section 11.10B relating to controlling access to closed systems to ensure data integrity.
AuditSafe supports either internal user accounts designed specifically to log in to the AuditSafe software or can be integrated with Windows Active Directory-managed accounts (via LDAP integration) for centralised password and account handling. This allows IT and QA/QC departments to either keep their 21 CFR/GMP-compliant system separate from their larger user network or integrate it directly with Windows Active Directory Management to handle things like password security, password resets, user access etc.
Users and their respective permissions can be grouped, allowing quick addition of new users as your team grows to an established group with pre-determined access defined by their role within the team. This simplifies onboarding processes and allows linking of permissions to job roles – for example all users defined as “analysts” or “supervisors” would have the same level of data access within the system.
AuditSafe forms the foundation of our OEM 21 CFR Part 11/GMP-compliant software development service which allows manufacturers to rapidly reduce time to market for their equipment/software solutions when selling into regulated markets. As part of this integration service we can also control user access to particular functions within the partner software to give even greater granularity over user access.

One of the most common questions regarding AuditSafe and our 21 CFR/GMP-compliant software more generally is “how is it better than a quality management system?”
A quality management system (QMS) is defined as a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. A QMS is a great way to coordinate an organisation’s activities to meet regulatory requirements and improve its effectiveness and efficiency continuously. A QMS is typically used as more of a file store and access repository for completed analysis reports rather than being capable of tracking data generation and analysis.
This leaves a huge hole in your audit trail – how can auditors check the validity of the raw data used to generate the analysis report that’s stored in your QMS?
AuditSafe is designed to integrate into your workflow before your completed reports are integrated with your QMS or LIMS – at the equipment and analysis level – to ensure that data is tracked, signed, auditable and secure from the moment it exists, throughout your analysis (performed within the system using your usual analysis software package) and finally to the exported analysis report, ready to be uploaded to your existing QMS.
Get in touch if you’d like to arrange a video call to discuss your specific workflow and how we can use AuditSafe to enhance your compliance with FDA 21 CFR Part 11, EU Annex 11, GMP standards or more! Or if you’re an equipment manufacturer or software developer interested in partnering with TotalLab to join the AuditSafe platform and allow your customers to use your products in a regulated environment you can find out more here.
Here at TotalLab we’ve been working with pharmaceutical customers for decades and we know that some of our users prefer using air-gapped computers to enhance security within their sites. This isn’t a problem, all TotalLab software can be activated easily either online or offline.
AuditSafe allows IT administrators and QC/QA managers to access all the electronic records and signatures for projects through one simple browser-based interface. Whether you have a single 21 CFR Part 11/GMP-compliant computer on-site to administer or one hundred, you can access project records, see which users are currently logged in and even see exactly which projects are currently being worked on!
Electronic records can be filtered by date range and downloaded in .PDF format allowing Auditors direct access to an entire facility’s records from one convenient access point.
Because TotalLab are not associated with any particular equipment vendor, we offer the potential to harmonise multiple different pieces of equipment and software all from different vendors within the AuditSafe system.
We know that compliance software is typically difficult to use, administer and requires extensive user training to implement correctly on-site. One of the unique benefits of the AuditSafe platform is that by bringing multiple pieces of equipment and software together in one compliant shell, lab users only need to be trained in one piece of compliance software. This massively reduces the risk of accidental non-compliance by users.
Auditsafe is TotalLab’s answer to one of the major pain points in introducing regulatory compliance in a manufacturing site – difficulty in integrating the solution within your existing workflow and network.
Here at TotalLab we’ve been creating and selling FDA 21 CFR Part 11/GMP-compliant software for the pharmaceutical and other regulated industries (such as hospitals) worldwide for over a decade with an incredibly robust and feature-rich software solution, however the hardest part of the installation and validation of the software has always been navigating the existing users highly complex and secure network/IT configuration.
From blocked ports to user permission headaches preventing end users from setting up the software themselves without their own IT professionals to hand, we thought there must be an easier way for our users to become compliant.
To this end, we can offer our AuditSafe software in three different configurations:
We wanted to create the easiest solution possible for our users to obtain and maintain secure regulatory compliance and minimise the potential for any incorrect usage of the software in a non-compliant manner. To do this we realised we needed to go beyond software and create an entire platform from the ground up, AuditSafe.
AuditSafe is a flexible product designed to integrate within your existing IT network.
It’s available in 3 different configurations:
Pre-Configured Server
connect to your existing IT network through a standard LAN cable. Once installed on site all that’s left to do is connect your computers to the AuditSafe and all audit trails, project files, original data etc. will be stored on the internal hard drives with internal backup
The AuditSafe Server arrives project-ready out of the box:
Cloud compliance offers a number of potential benefits to the user including:
However it does have some major risks associated:
Learn how TotalLab's software implements secure electronic records, robust audit trails, and user authentication protocols. Achieve compliance seamlessly with our expert guidance
Read More >
The Good Manufacturing Practice (GMP) regulations that govern pharmaceutical and medical device manufacturing can seem overwhelming, use this handy guide to stay compliant!
Read More >
Discover the essential ALCOA principles in life sciences and pharmaceutical production. Learn how Attributable, Legible, Contemporaneous, Original, and Accurate guidelines ensure data integrity, regulatory compliance, and patient safety. Understand the extended ALCOA+ principles and their critical role in the industry
Read More >
We have created a 21 CFR part 11/GMP compliance guide to explain how our AuditSafe software helps fulfill all the requirements of FDA 21 CFR Part 11/GMP legislation and can help you achieve compliance
Read More >
This guide provides on how you can use AuditSafe can ensure your electronic signatures are generated in compliance with 21 CFR Part 11/GMP regulations.
Read More >
Read our 21 CFR part 11/GMP compliance checklist to ensure you have the right checks in place to comply with the regulations
Read More >