Case Studies
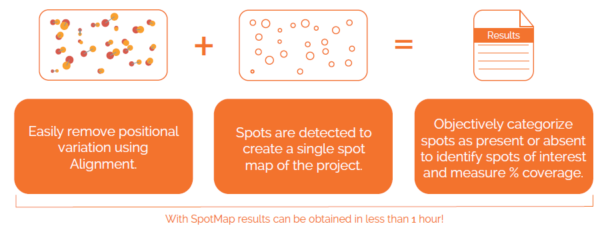
Case Study: Western Blot Assessment of Polyclonal Anti-Host Cell Protein Antibody Production, Covance BioCMC (Now LabCorp)
Case Study: University of Aberdeen, Proteomics Core facility
“The proteomics core facility provide protein analytical services to academics and companies, mostly in the local area but also worldwide. We offer a wide range of services to try and accommodate any needs, these can vary from simple protein identifications to large scale gel/mass spec experiments. Currently we are mainly offering services in: Gel electrophoresis (1D, 2D, DIGE), In-depth proteome analysis (Q-Exactive LC-MS), molecular histology (MALDI- imaging), protein identification/quantification, mapping of protein modifications and rapid microbial identification (MALDI Biotyper).”

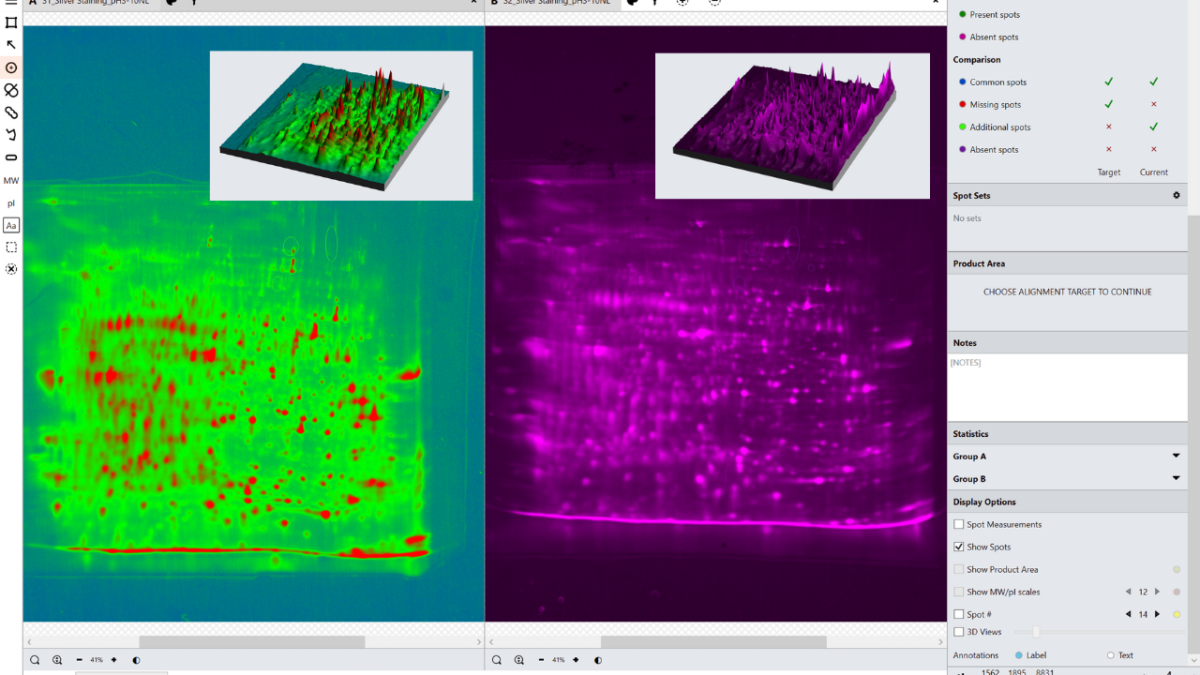
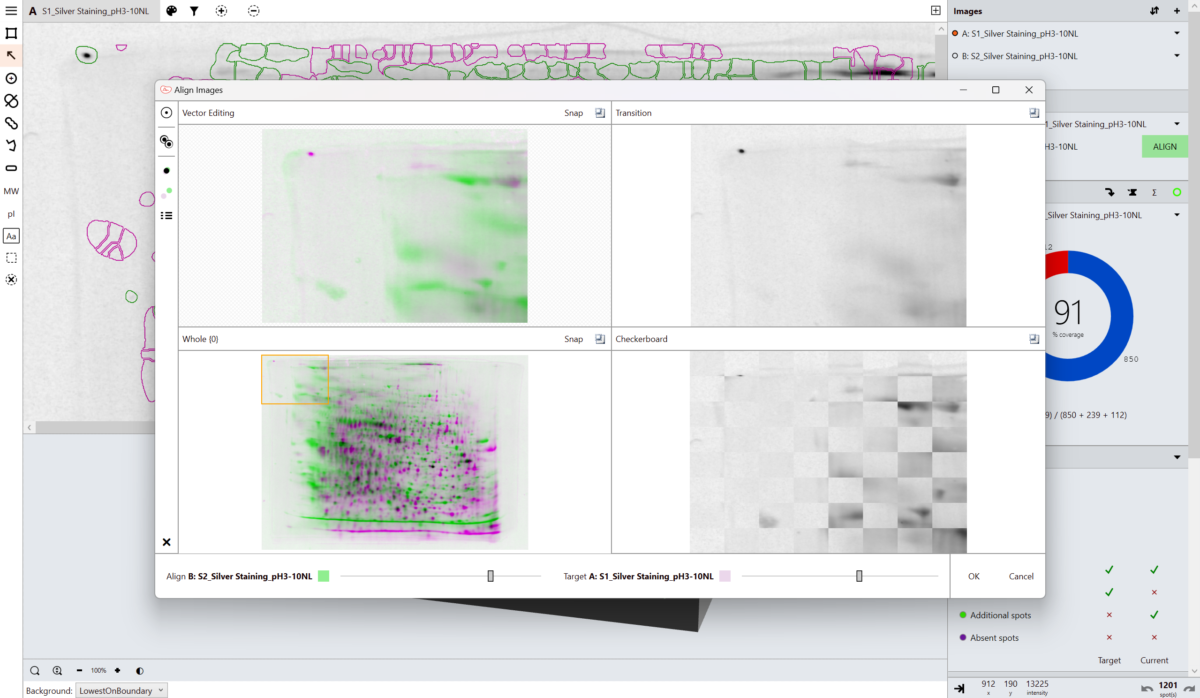
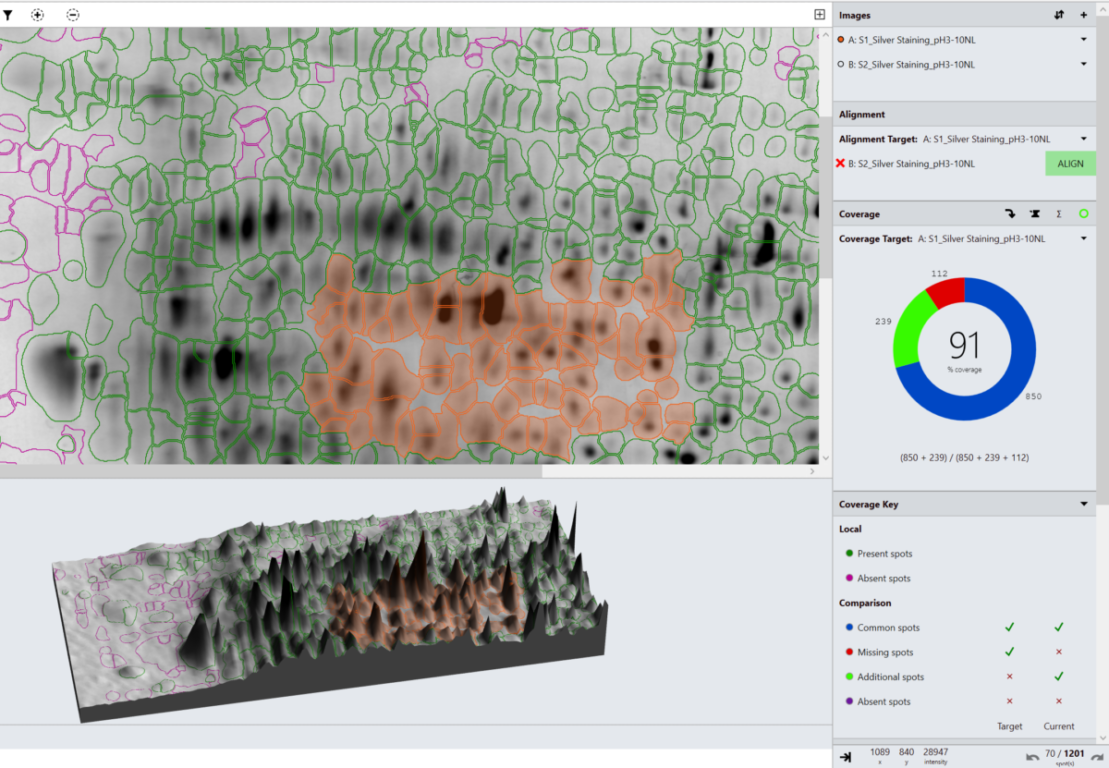
“We have had real diffculty matching reactions on blots to the corresponding areas on the gels. With our previous technology it genuinely wasn’t possible so adding the SpotMap software to our facility has helped greatly. Because a big reaction on blots doesn’t necessarily mean a big amount of protein on the gel it’s sometimes hard to match patterns by eye. In the past we have had to rely on matching patterns by eye and end up cutting out the wrong area of the gel. Once we had the images put through the SpotMap software it corresponded to areas that personally I would have never matched (which ended up being the correct areas).”
Craig Pattinson, Research Technician, Proteomics, Aberdeen University
Case Study: Rockland Immunochemicals
Poster, originally presented at the WCBP 2016 conference: “2D-SDS PAGE Analysis Methods For Determining HCP Coverage Using Process Derived anti-HCP Polyclonal Antibodies”

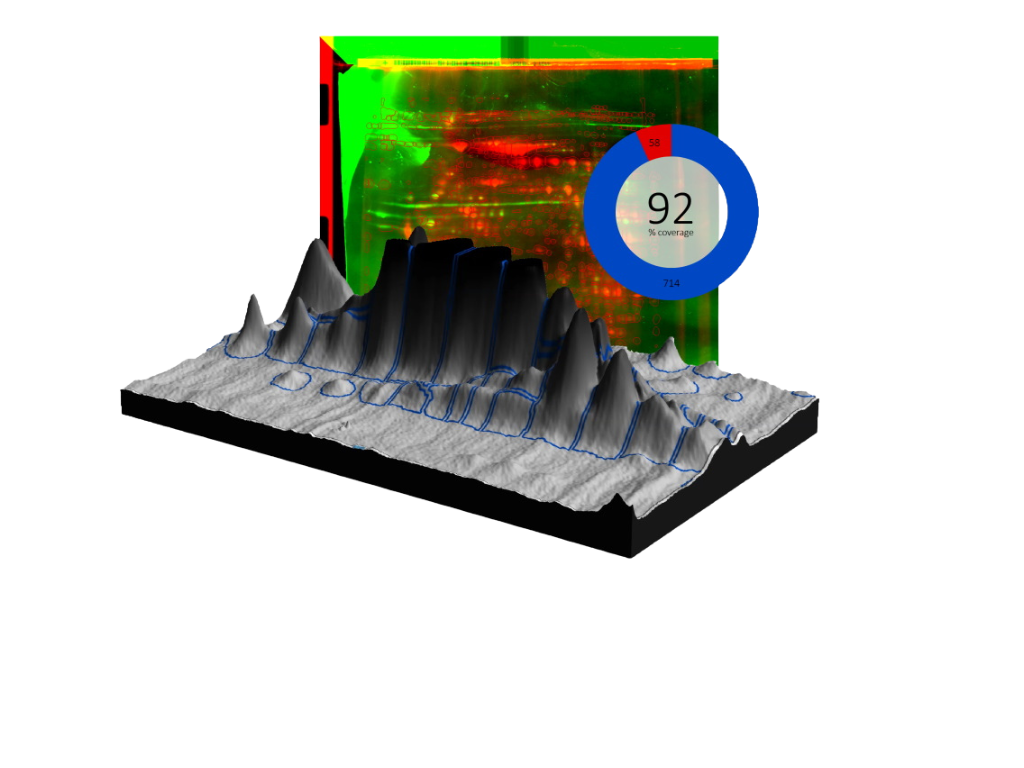
“Both spot analysis methods generated a final coverage that was between 44 – 59%. However there was much more variation in coverage between the 1.0 second exposure and 5.5 second exposure (11%) using the PD Quest Spot analysis. Spot analysis using SpotMap resulted in coverage that was consistent within 1% between all three blot exposure times. Workflow between the two analysis systems varied by about 2 hours – analysis took approximately 1 hour using the SpotMap software and approximately 3.5 hours using the PD Quest software. PD Quest spot analysis software offers numerous options and parameter selection criteria whereas SpotMap software has limited parameter selection but requiring fewer steps in the workflow to determine antibody coverage.”
Case Study: Minimising Inter-User Variation in Coverage of 2D Gels
This report demonstrates the inter-user variation of SpotMap used by three individuals of varying experience on three data sets categorised as hard,
medium and easy. Results obtained were within a range of 10% of each user, even when the most challenging images were used. Key areas of the analysis
where variation may be introduced have been identified.
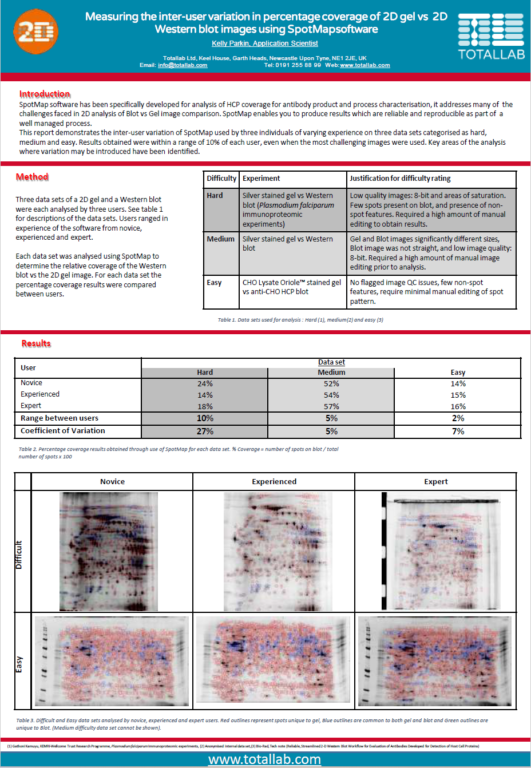
“Typically with 2D gels and Western blots the biggest source of variation is the image quality. The automatic image QC flagged two of the data analysis results as being potentially imprecise due to the use of 8-bit images. Smearing/steaking, saturation and presence of non-spot features also affects analysis precision.
There are areas of the analysis that require particular consideration to improve objectivity and precision. Within the software, image editing, alignment vectors added, spot detection parameters used and individual decisions on the presence of a spot will all impact the results.
However, SpotMap gives reproducible and reliable results within a range of 10%, even using challenging images. The use of high quality images which enable a consistent analysis approach between users will reduce the variation, as seen in the easy data set where results are within a range of 2%. Production of an SOP that addresses all aspects of the analysis, including optimised gel running and image capture would increase the objectivity of the results, reducing the subjective decisions required during analysis.”
Case Study: Rockland Immunochemicals
Poster, originally presented at the WCBP 2018 conference: “Image analysis used to minimise inter-user and inter-lab variation of results
from measuring HCP-antibody coverage by comparing features between 2D gel and 2D Western blots.”