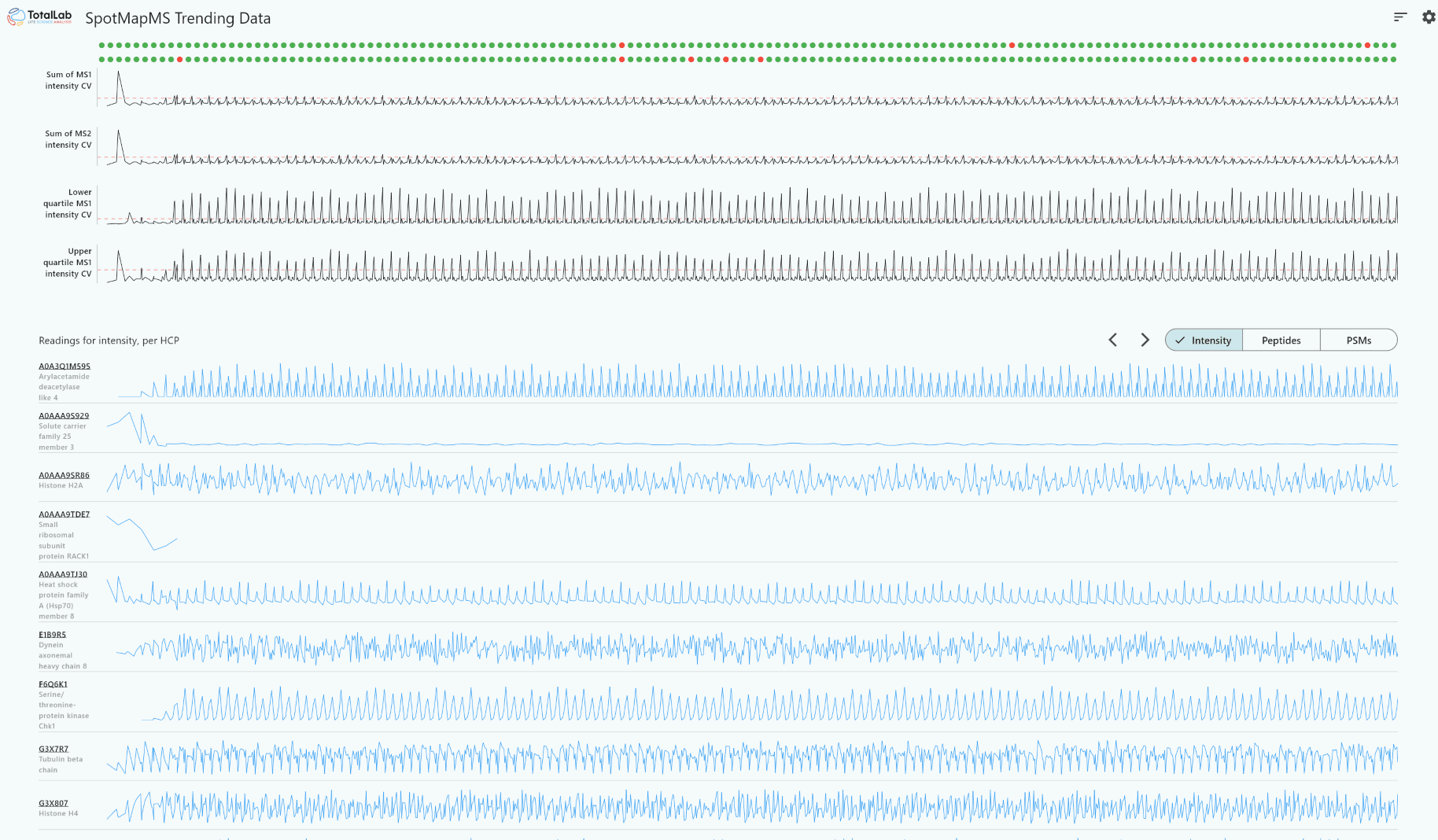
Solving Current Challenges in LC-MS based HCP Analysis
SpotMap MS is a brand-new software and database package specifically designed from the group up for HCP analysis via LC-MS. It’s fully automated, simply specify the location of your FASTA file and raw mass spec trace files and it can generate a completely automatic HCP analysis. Utilizing TotalLab’s AI-powered HCP database, it can reduce the output of an analysis to feature only those HCPs that have been identified in peer-reviewed publications to be potentially dangerous and assign a threat level to them.
SpotMap MS solves current challenges preventing the wide scale adoption of HCP analysis via LC-MS:
- Complex software. Difficult to drive and time-consuming, preventing users from performing the routine calibration and maintenance they need to do to guarantee accuracy within their specific LC-MS equipment
- Software designed for Proteomics. Current LC-MS analysis software is designed for large-scale proteomic use, not for identifying HCPs. It’s over complicated for the task at hand when you really want to answer a simple question – does my sample contain HCPs? and if so, are they dangerous?
- Lack of reproducibility. Other software packages focus on dealing with many different research questions, and to do so require large numbers of parameters. This compromises the reliability of that data as it is too easy for different users to get different answers. SpotMap MS is designed from a “QC first” perspective designed to align with best practices in the field of HCP analysis.
- Lack of automation. Despite the maturity of LC-MS as an analytical technique, data analysis still remains a time-intensive and laborious process. SpotMap MS is designed as a “set it and forget it” solution automating your analysis and dropping you straight into a comprehensive HCP report once completed.
- Very few 21 CFR/GMP-compliant options. The limitations listed above have so far been a barrier to LC-MS’s adoption within regulated environments, with very few manufacturers supporting 21 CFR/GMP-compliance, limiting user choice. SpotMap MS is fully compatible with our AuditSafe software to achieve 21 CFR/GMP-compliance built by the industry, for the industry.
The software has been developed in collaboration with and in response to pharmaceutical industry feedback and the world’s leading experts in HCP analysis from the BEBPA Host Cell Protein Conference of which TotalLab has been a long time supporter.
It is easy to master, with accurate results obtained quickly and reproducibly.
Why Choose SpotMap MS?
SpotMap MS is specifically designed from the ground up to automatically analyze LC-MS traces for host cell proteins – rather than being repurposed proteomics based software like competitor solutions. It does this by comparing LC-MS traces to a user provided FASTA file and so relies on data dependent acquisition (DDA) methodology. This allows users to either import their own, proprietary, existing spectral library of identified host cell proteins or use a commercially or publicly available one.
SpotMap MS also includes TotalLab’s AI powered HCP identity spectral library that includes associated HCP threat level, so even if you’re just getting started in the world of LC-MS based HCP analysis you won’t be working blind.
SpotMap MS has been built and continues to be developed in direct contact with the world’s largest pharmaceutical companies performing this work on a day to day basis. By working with the world’s leading experts on LC-MS HCP analysis TotalLab has created a software tool developed for scientists, by scientists.
A New Approach to Host Cell Protein Analysis
As SpotMap MS is designed from the ground up for HCP analysis, rather than adapting an existing piece of proteomics software for the task, it works slightly differently to other software in this space. We set our analysis parameters (for example, false discovery rate (FDR)) to be extremely sensitive automatically within the software and then filter the HCPs we detect using our proprietary database of already identified potentially dangerous HCPs.
This shows you everything – no data reduction or hidden data behind the scenes. Any host cell proteins we even suspect of being being both present and risky we highlight to the user. From there, we then provide facilities for the user to manually verify those hits as quickly and comprehensively as possible. We’ve put a lot of effort into making this verification process fast and reliable, with a guided step through of everything you’ll be reporting on, and a record of what has been inspected so you can show regulators that it has all had human eyes on verification.
We take this approach of risking generating false positives (and being able to eliminate those quickly within the software) as we believe that it is far preferable to do it this way than to risk false negatives – the risk of a dangerous HCP “getting through” undetected. This is because HCPs such as enzymes can cause serious problems even when only present in vanishingly small amounts (which could be close to the limits of detection of your LC-MS system).
And because you don’t need to worry about HCPs that have not been reported in the literature as potentially dangerous, the human effort required to make this happen is much less than you might imagine.
“Hands Free” Automatic Analysis of LC-MS Traces
SpotMap MS is designed to be as automated as possible to fit in with your life and your workflow. This allows you to save as much time as possible when analyzing LC-MS traces, through advanced “hands-off” automation that allows you to “set and forget” your analysis through to unique upfront data visualization, allowing you to make a quick upfront decision as to whether a dataset is worth investing analysis time into. If a technical error has occurred in processing, you still have time to prepare a new sample to be analyzed, rather than only realizing at the end and wasting huge amounts of time and potentially your sample.
SpotMap MS achieves this by allowing a user to assign a “watched” folder. As soon as traces hit that folder they’re automatically analyzed per a set of pre-configured parameters by the user.
There’s also provision for the software to restart an automated analysis at the last place it reached just in case anything should happen to the computer mid-operation. No more being chained to your desk waiting for your analysis to finish!
LC-MS Software with Data Quality at its Core
As Scientists, we want to be confident that we are investigating real trends and ensuring our data is of a high standard is central to this. Being warned early about acquisition failures saves users both time and money. SpotMap MS has a strong focus on making data quality checks automatic and easy to to perform. A new, comprehensive method known as the Protein Verification Loop has been developed by TotalLab specifically for SpotMap MS to ensure that your entire analysis is reliable; from raw data all the way to protein quantification.
This kind of process is specifically mentioned in the latest regulatory guidance, USP General Chapter 1132.1 states: “Search engine output should not be trusted implicitly, especially for low-abundance HCPs”. “Manual inspection of peptide-level raw data (XIC trace, MS spectrum, and MS/MS spectrum) should be a routine part of HCP data analysis process”
Our data quality metrics guide user actions, are easy to interpret and ensure that users at all experience levels can be effective software users and HCP mass spec experts without extensive training.
In other words: the methods and experience of an expert in HCP detection via LC-MS is now codified in an application for you to use
Metrics measured using this method can be used in System Suitability Testing, method development and to characterize the behavior of your HCPs in your mass
Free, Built-in HCP safety-profile Database
Integration with a Host Cell Protein safety-profile database saves users research time and facilitates prioritization of potentially dangerous HCPs.
As mentioned above, SpotMap MS includes TotalLab’s AI powered HCP identity spectral library that includes associated HCP threat level, so even if you’re just getting started in the world of LC-MS based HCP analysis you won’t be working blind.
This database has been generated with the latest techniques in AI, evolves over time and lists the known current threat level of HCPs.
Or if you have your own existing HCP database you wish to use, SpotMap MS also supports integration of existing datasets.
Integration with Trendlab
SpotMap MS experiments can be tracked over time with Trendlab, another exciting new product from TotalLab that enables longitudinal tracking of your MS experiments. This extended functionality allows you to:
- Track your system suitability tests before and after each run, maintaining a long term record of your equipment health, setup and calibration.
- Monitor HCP content across multiple runs and observe any changes in HCP content that might indicate issues or changes in your workflow
- Share HCP content data across different departments on-site to track purification processes
21 CFR Part 11 and GMP/GMP-compliance
SpotMap MS, along with all TotalLab software, can be integrated into our 21 CFR Part 11/GMP-compliant AuditSafe software to ensure full 21 CFR/ALCOA/GMP/EU Annex 11 compliance for use in regulated industry environments (such as the pharmaceutical industry) where data traceability, accountability, and integrity are critical.
AuditSafe restricts software and data access through secure user sign-ins, granular permissions, audit trails, electronic signatures and data authenticity checks. Click here to find out more.
Full IQ/OQ Validation Packages Available
At TotalLab, we’ve been supporting the world’s largest pharmaceutical companies with highly secure, production-ready versions of our software for over a decade now and enjoy very good relationships across the industry.
We know the importance of properly validated software and how it forms part of your QC journey so we offer a full, in-person IQ/OQ package to accompany the sale of regulated versions of our software

Case Studies (Click to Enlarge)
Developing a High-Throughput Host Cell Protein Analysis Workflow with Novel SpotMap MS Software – Presented at CASSS Mass Spectrometry Conference 2025
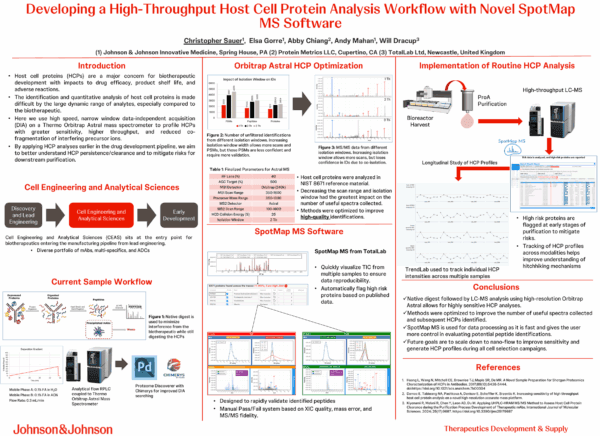
“Spotmap MS is used for data processing as it is fast and gives the user more control in evaluating potential peptide identifications”
Chris Sauer, Johnson & Johnson
The Total HCP Project with Novel SpotMap MS Software – Presented at BEBPA HCP Conference 2024

Flexible Licensing Options
At TotalLab we’ve been working with pharmaceutical and academic life science customers for decades, we know that some of our users prefer offline licensing to enhance security within their sites. This isn’t a problem, all TotalLab software can be activated easily either online or offline.
Our licensing system licenses a whole computer at once, with no restrictions on the number of users who have access to the software on that computer.
Licenses can be purchased singularly for one computer or we also offer discounts on multiple license purchases at the same time if you want to cover multiple computers across your site; enabling hybrid working to perform analysis outside of the lab or to allow multiple analyses to happen at the same time.
We can also support enterprise licensing for full site-wide licenses covering large pharmaceutical manufacturing facilities and research facilities at a significant discount vs buying singular or multiple licenses.
For pricing and any other licensing related queries please contact us.
Support and Maintenance
At TotalLab, we’re the trusted partner of some of the world’s largest pharmaceutical companies, so we understand the importance of support and maintenance contracts for software, especially in time-critical and high-value processes.
We take pride in offering the highest level of support and educational resources to all of our customers. Our team of PhD-educated life science experts are dedicated to ensuring that you have the best experience possible all whilst understanding the scientific principles behind your experiments. Pricing for unlimited technical support, training and free updates to the software are included in your license cost – one fee, complete piece of mind. You’ll always be eligible for the latest version of the software on the latest operating systems so there’s no fear of future operating system incompatibility or running insecure, outdated software for critical operations.
We never use call centers or ticketing systems – when you need support you’ll speak directly to a member of the TotalLab team. We understand your time is valuable, which is why we aim to resolve your queries as quickly as possible, ensuring minimal disruption to your workflow.
In addition to our exceptional support, we also offer a range of educational resources. Our extensive library of tutorials and guides will provide you with the knowledge you need to use our software effectively. Whether you are a seasoned professional or a newcomer, our resources will ensure that you have the skills you need to succeed.
You can access our video tutorials here:
If you have a question about using our software, you can email support@totallab.com and a member of our team will be in touch to help you.
How to Purchase
The best way to ensure you’re purchasing the latest version of SpotMap MS with the highest level of technical support is directly from us here at TotalLab by using the contact us form. We can accept payment in GBP (£), USD ($) or EUR (€).